Passivation is a process that enhances the corrosion resistance of metals by creating a protective oxide layer on their surface. This layer acts as a barrier, preventing further oxidation and deterioration. Passivation is commonly applied to stainless steel, aluminum, and other alloys, often using chemical treatments or electrochemical methods.
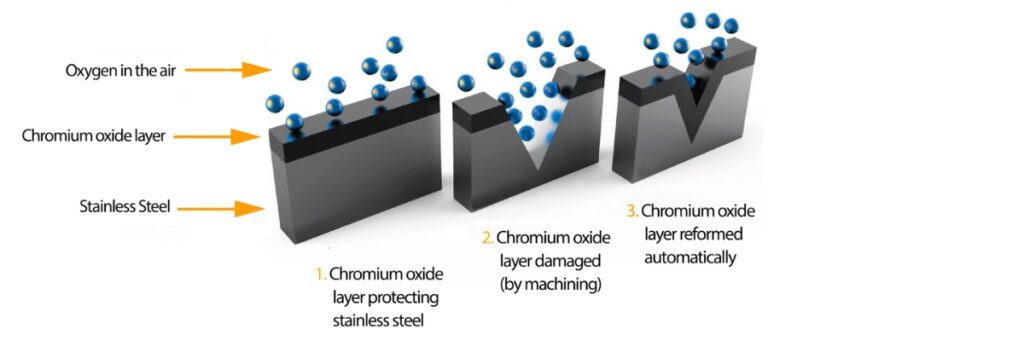
In the case of stainless steel, for example, the passivation process typically involves cleaning the metal to remove contaminants and then exposing it to a passivating solution, often containing acids like nitric acid. This helps to enhance the natural oxide layer, improving the metal’s durability and resistance to rust and corrosion.
Overall, passivation is crucial in various industries, including aerospace, automotive, and food processing, where the longevity and integrity of materials are essential.
Corrosion Potentials Created During Fabrication
Defects and contaminants that can lead to corrosion are caused during the manufacturing and fabrication process. Surfaces must be cleaned of the following corrosion potentials:
- Embedded iron particles will be picked up on forming rollers, carbon steel wire brushes, layout and cutting tables, and grinding.
- Heat tint: welding heats the base metal causing heavy oxide films (scale) to develop in the area of applied heat. The oxide film range in colour from straw yellow to black. The colour variation in the base metal is also dependent on the amount of oxygen present during the welding process. Heat tint will result in lower corrosion resistance of the stainless steel.
- Weld flux is produced by welding with covered electrodes and forms along the sides of the weld bead. Weld flux is difficult to remove requiring brushing with stainless steel wire brushes, abrasive disc and flapper wheel grinding. These methods may leave small flux particles at the side of the bead head. The flux particles are excellent crevice formers.
- Arc strikes and spatter: arc strikes produce small pinpoint surface defects in the protective film, as does weld spatter, that become areas of corrosion.
- Scratches and paint: deep scratches also initiate corrosion, as can paint, crayon marks, and other instruction markings if they are not removed.
Passivation Process
- Mechanical Cleaning
- There are many mechanical methods used to clean welds such as chipping, brushing, grinding and blasting.
- Degreasing and Inspection
- Passivation cannot form or enhance the protective film when grease, oil, fingerprints, or other organic contamination are present on the product contact surface.
- Degreasing and general cleaning may be accomplished by immersion in, swabbing with, or spraying with alkaline cleaner, solvent or detergent cleaners or a combination of these; by vapour degreasing, by ultrasonics using various alkaline cleaners; by steam, with or without cleaner or by high pressure water-jetting.
- Inspection by water-break test to determine the oil, grease, and other organic contaminants are completely removed from the surface.
- The copper sulphate and ferroxyl tests are used to determine the present of iron.
- Passivation (immersion or spraying)
- The use of nitric acid has two purposes. Firstly, the acid dissolves any high carbon tramp steel. Secondly, it assures a uniform, clean surface that results in the consistent formation of the passive chromium oxide film.
- Rinsing
- Immediate and thorough rinsing in water, pH 6 to 7, is critical. Immersion, neutralization, and rinsing must be completed without allowing surfaces to dry between steps.
Note:
It is recommended you contact your CIP engineering partner to carry out the passivation process.Reference: Maller, R. R (1998). Passivation of Stainless Steel. EHEDG Doc. 18.
